Quality standards
At Bedrocan, we produce medicinal cannabis following the highest quality standards. Our whole production process is under strict quality control. This is essential for delivering a product suitable for patient use, scientific research and product development.
For cultivation, we follow our guidelines for Good Medicinal Cannabis Cultivation Practice (GMCCP). From drying to packaging, we follow the EU Good Manufacturing Practice (GMP) guidelines, for which we are also officially certified.
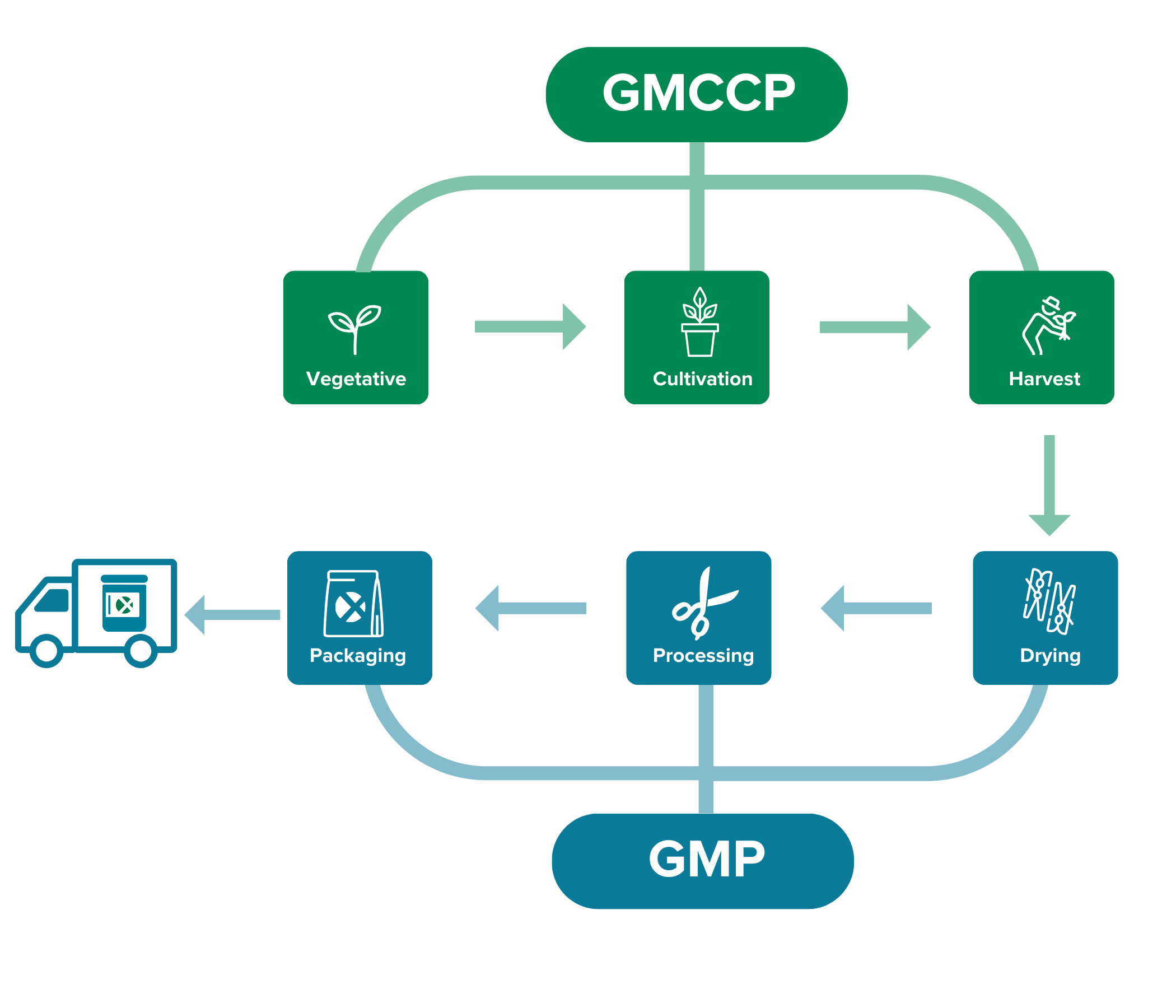
GMCCP- Good Medicinal Cannabis Cultivation Practice
Bedrocan goes beyond the European Good Agricultural and Collection Practice (EU-GACP) requirements in order to produce pharmaceutical-quality products. At the moment, the EU-GACP is the only standard required by regulatory authorities within the EU for the cultivation of medicinal cannabis. However, Bedrocan considers the EU-GACP guidelines not sufficient for cannabis intended for medicinal and scientific use. That is why we have developed our own guidelines for Good Medicinal Cannabis Cultivation Practice (GMCCP).
Bedrocan’s GMCCP adopts many GMP principles and accounts for the complexity of cultivating the cannabis plant for medicinal and scientific use. It provides a system for managing quality in cultivation and the production of cannabis within a fully controlled indoor facility. All Bedrocan facilities diligently follow the GMCCP guidelines.
GMP- Good Manufacturing Practice
Good Manufacturing Practice (GMP) is critically important to ensure medical products are consistently produced and controlled according to defined quality standards. Bedrocan was the first cannabis company in the world to obtain a GMP certification. Our GMP-approved production process includes the following:
- Drying
- Processing
- Bulk packaging and labelling