A clinical research review about cannabis for prescribers
Over the last 20 years a number of clinical studies and scientific research projects have been performed on the Bedrocan® medicinal cannabis product. This clinical research review about cannabis for prescribers is a summary of the most important findings.
Pain – opioid-sparing treatment option
Bedrocan® may be used as part of an opioid-sparing strategy in the treatment of pain. In this study of pain clinic patients, 78.5% were prescribed an oral dose of Bedrocan®, with most patients receiving a prescription for 12 months (range 8-21 months). At study initiation, 20% of the cohort were prescribed high doses of opioids (> 90 mg morphine equivalent/day). A high benzodiazepine prevalence was also observed. After 6 months of cannabinoid therapy, a significant increase in the number of patients not receiving opioid medications was identified (from 32.1% to 55.4%).
However, a decrease in anticonvulsant, antidepressant, and benzodiazepine medications did not occur.
Study reference: Nunnari et al. (2022). European Review for Medical and Pharmacological Sciences.
Pain – no additional respiratory depression
Respiratory depression is a common side effect of strong opioids. The co-administration of inhaled Bedrocan® (100 mg cut flos) has no effect on ventilatory control after pre-treatment with placebo or oxycodone (20 mg) in a randomised controlled crossover trial in healthy individuals.
The inhaled Δ-9-THC dose, taken at 1.5 and 4.5 hours post the oral oxycodone dose, did not enhance opioid induced respiratory depression. Oxycodone itself reduced isohypercapnic
ventilation (VE55) by 30% from baseline. The second cannabis inhalation had no effect on VE55. The coadministration of oxycodone and Δ-9-THC increased the degree of sedation by up to 30% and mild intoxication by up to 10%, and decreased energy state by up to 30% and the state of alertness by up to 40%.
Study reference: Van Dam et al. (2023). British Journal of Anaesthesia.
Bedrocan®
Bedrocan® is the brand name of the variety Cannabis sativa L. ‘Afina’. Bedrocan® has a consistent composition of active ingredient, batch-to-batch, dose-to-dose. Standardisation allows prescribers to better monitor dosing, condition progress, and minimise side effects.
A high-quality vaporizer device is an effective inhalation delivery system. It makes it possible to titrate to an optimal dosage (e.g. Storz & Bickel). Administered by inhalation it may have positive effects on subjective pain intensity in a variety of pain-related medical conditions, with most research focussing on neuropathic pain. Further randomised controlled trials are needed to demonstrate safety and efficacy.
Fibromyalgia – an alternative treatment
Bedrocan® may represent an alternative analgesic for patients diagnosed with fibromyalgia but who do not respond well to conventional treatments (i.e. tramadol, amitriptyline,
duloxetine, pregabalin).
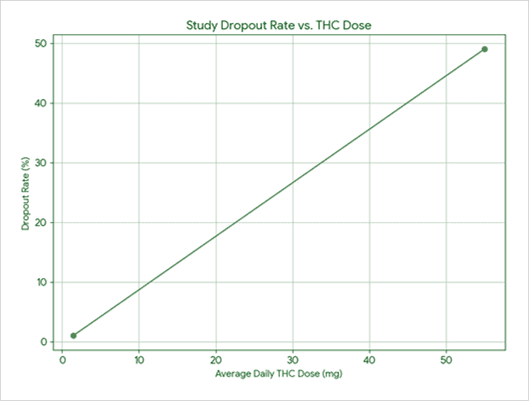
This retrospective study showed this cohort of pain clinic patients responded well to Bedrocan® 200 mg/day cut flos. A reduction in pain intensity was found at 1, 3, and 12 months of treatment. Significant improvements were observed in the Numerical Rating Scale (NRS), Oswestry Disability Index (ODI), Hospital Anxiety and Depression Scale, Widespread Pain Index (WPI), Severity Score (SyS) at 1 month; in NRS, ODI, and WPI at 3 months; and in NRS, ODI, and SyS at 12 months.
Most patients treated with Bedrocan® continued their therapy. Stopping therapy was due to nonserious, transient side effects such as confusion and dizziness. An appropriate titration period would help reduce the incidence of these side effects, and improve medicine efficacy and treatment compliance.
Study reference: Mazza. (2021). Journal of Cannabis Research.
Avoiding side effects
Often CBD (cannabidiol), a non-intoxicating cannabinoid, is prescribed to attenuate the psychotropic side effects of Δ-9-THC. A recent study, however, shows CBD does not influence any of the cognitive impairments or psychological symptoms. Neither does CBD attenuate any of the other acute effects of Δ-9-THC, including behaviour, subjective experiences, and cognition.
Additionally, co-administration of CBD had no influence on Tmax, metabolites OH-THC or COOH-THC, or to total systemic exposure (AUC). A recent study demonstrated that CBD after a 450 mg oral dose of CBD can increase the acute effects of Δ-9-THC, likely explained by CBD inhibiting cytochrome P450-mediated metabolism of THC.
Study references:
- Chester et al. (2022). Journal of Cannabis and Cannabinoid Research.
- Lawn et al. (2022). Addiction.
- Englund et al. (2022). Journal of Neuropsychopharmacology.
- Gorbenko et al (2024). Clinical Pharmacology & Therapeutics.